| 2005 |

|
YEAR BOOK |
National University of Ireland, Galway
|
Researching metal catalysts in the Department of Chemistry
|
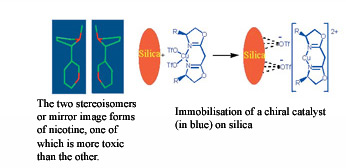
In the body seemingly very similar molecules can have dramatically different effects. When a molecule has a mirror image that is different it is said to be 'chiral'. Chiral molecules are related in the same way as a right and left hand are mirror images. Our bodies are made from a huge variety of chiral species. How a chiral molecule in the body will act depends on the chiral species it meets. Examples of such different interactions in the body are numerous, one of the two mirror images (or stereoisomers) of limonene smells of oranges and the other of lemons, one stereoisomer of ibuprofen is an analgesic drug whereas the other is inactive and one stereoisomer of nicotine is more toxic than the other.
Pharmaceutical companies, on identifying a drug, need to check the activity of all the stereoisomers in the body and seek to synthesise only the required active ingredient. The preferential synthesis of one stereoisomer of a molecule can be a difficult task.
We are engaged in research seeking to generate techniques which are generally applicable to the preferential synthesis of one stereoisomer. This is achieved using a catalyst which controls the reaction. Typical catalysts consist of a metal component which becomes the centre where the reaction takes place and a molecule (or ligand) which surrounds the metal and gives control of the chirality of the product.
|
|
Contact: Dr Patrick O'Leary,
Department of Chemistry, NUI Galway;
Tel: +353 (0)91 492476; E-mail: [email protected]