| 2005 |

|
YEAR BOOK |
National University of Ireland, Galway
|
Research at NUI Galway asks how cells keep their DNA intact
|
Cells must make sure that their DNA does not suffer damage, as such damage can be lethal or lead to changes in how cells behave, potentially causing cancer. A number of complex mechanisms have evolved to make sure that DNA remains intact in the face of numerous environmental challenges, such as ultraviolet light, DNA-damaging carcinogens found in cigarette smoke or ionising radiation that is used in radiotherapies. Understanding how these cells maintain their genomes may allow us to improve our knowledge about how to kill tumour cells. These mechanisms and the proteins involved in them are the subject of the work carried out by researchers in the Genome Stability Cluster, in the Department of Biochemistry/National Centre for Biomedical Engineering Science at NUI Galway. Currently, some thirty-five researchers, from sixteen countries, carry out research in the Genome Stability Cluster. At present, four research groups make up this cluster, with additional recruitment underway.

Dr. Michael Carty's group is investigating the link between replication arrest induced by DNA damage, and activation of cell cycle checkpoints in human cells. As a model system, they focus on the role of DNA polymerase eta (pol eta) in DNA replication following DNA damage. The gene encoding pol eta, RAD30, is mutated in the human disease, xeroderma pigmentosum variant (XPV), causing a 1000-fold increase in the incidence of UV-induced skin cancer. Carty's researchers are examining the role of pol eta in activation of cell cycle checkpoints controlled by the DNA damage-responsive kinases, ATM and ATR, and DNA damage-induced phosphorylation of known ATM and ATR substrates, the 34 kDa subunit of replication protein A (RPA), CHK1, and p95 (NBS1/Nibrin). They are also applying functional genomics approaches to identify polymorphisms in the human RAD30 gene that may confer an increased risk of developing skin cancer. Recent findings made in the Carty lab have shown that RAD30 expression is important in determining the sensitivity of human cells to cisplatin, an important chemotherapeutic drug.
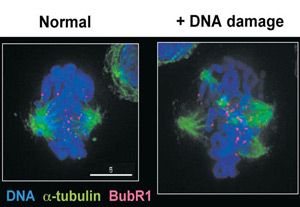
One aspect of research in SFI Investigator Dr. Ciaran Morrison's lab asks how chromosome damage signals are transmitted in vertebrate cells, by disrupting telomere structure using gene targeting and by inducing DNA double-strand breaks at defined chromosomal loci. Following up on their recent results that linked DNA damage with centrosome amplification, Morrison's group is characterising the kinetics of such amplification following ionising radiation treatment. Researchers are also dissecting the roles of the key DNA damage-responsive ATM and ATR kinases and the downstream Chk1 kinase in centrosome amplification. In addition, Morrison and co-workers are using a biochemical/ proteomic screen for novel chromosomal substrates of the DNA-dependent protein kinase. They hope that these approaches will allow them to obtain new insights into the interplay between these DNA damage-responsive systems in vertebrate cells.
|
|
Contact: Prof. Noel F. Lowndes,
Tel: 091-49 27 06; E-mail: [email protected]
Web: http://www.nuigalway.ie/faculties_departments/biochemistry/