 0.07%
k-carrageenan microgels in 200mM KCl
In recent years, there has been an
explosion in the development and market for "functional foods", i.e. foods that have been "fortified" with vitamins, minerals or health-promoting micro-organisms. Because of their inherent instability, these nutrients and bacteria need encapsulation in matrices such as microgels (gel particles 1-1000mm) for their delivery to any specific site within the digestive tract. Microgels have been produced from matrices of gums such as k-carrageenan, alginate, gellan and xanthan gum-based beads. k-carrageenan comes from marine algae and exists as random coil chains at high temperatures with a coil-to-helix transition occurring on cooling below a certain temperature. This is directly related to cation concentration with K+ ions being more efficient than Na+ or Ca++ ions for stabilising the helix state and promoting gelation. Electrostatic attraction occurs between the negatively charged sulphate groups of k-carrageenan and a predominantly positive region on k-casein in milk. This contributes to the functionality of k-carrageenan in products such as milk fat emulsions, desserts, ice cream and chocolate milk.
0.07%
k-carrageenan microgels in 200mM KCl
In recent years, there has been an
explosion in the development and market for "functional foods", i.e. foods that have been "fortified" with vitamins, minerals or health-promoting micro-organisms. Because of their inherent instability, these nutrients and bacteria need encapsulation in matrices such as microgels (gel particles 1-1000mm) for their delivery to any specific site within the digestive tract. Microgels have been produced from matrices of gums such as k-carrageenan, alginate, gellan and xanthan gum-based beads. k-carrageenan comes from marine algae and exists as random coil chains at high temperatures with a coil-to-helix transition occurring on cooling below a certain temperature. This is directly related to cation concentration with K+ ions being more efficient than Na+ or Ca++ ions for stabilising the helix state and promoting gelation. Electrostatic attraction occurs between the negatively charged sulphate groups of k-carrageenan and a predominantly positive region on k-casein in milk. This contributes to the functionality of k-carrageenan in products such as milk fat emulsions, desserts, ice cream and chocolate milk.
A study was undertaken at Moorepark to examine the effects of k-carrageenan microgels on the rheological properties of a model yoghurt system. Instantaneous k-carrageenan microgels were formed by adding 0.7% k-carrageenan drop-wise at 80�C to a KCl solution or high-heat reconstituted skim milk solution at 22�C with shearing for 5 min at 6000rpm using a Silverson� mixer. The temperature of the dispersion was maintained at 22�C during shearing by placing the beaker in iced water. An additional sample was prepared by adding the k-carrageenan to the reconstituted skim milk solution at 60�C with cooling in the absence of shear. Development of yoghurt structure was measured using dynamic oscillatory rheology on acidification at 40�C to pH of 4.6 in 120 min using an acidulant (glucono-d-lactone) with determination of the elastic modulus (G').
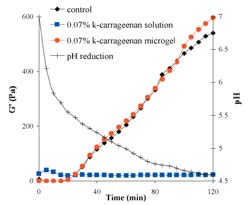 Acid gelation profiles (40�C) of 5% heated skim milk protein (u), or 5% heated skim milk protein with 0.07% k-carrageenan added at 60�C (n) or as microgels (l). Typical pH profile (+) is included.
Stable microgels were generated in the KCl and 5% reconstituted milk solution with particles sizes of 40-100mm. 5%
reconstituted skim milk showed an increase in G' with increased acidification, with gelation commencing at pH 5.5 and a strong gel with a G' of 540Pa was obtained at pH 4.6. The inclusion of 0.07%
k-carrageenan solution prevented gelation on acidification but the sample with 0.07% k-carrageenan present as microgels had similar rheolgical behaviour to the control system. This indicates that k-carrageenan microgels have the potential for use as delivery systems in yoghurt without having detrimental effects on the rheology of the yoghurt.
Acid gelation profiles (40�C) of 5% heated skim milk protein (u), or 5% heated skim milk protein with 0.07% k-carrageenan added at 60�C (n) or as microgels (l). Typical pH profile (+) is included.
Stable microgels were generated in the KCl and 5% reconstituted milk solution with particles sizes of 40-100mm. 5%
reconstituted skim milk showed an increase in G' with increased acidification, with gelation commencing at pH 5.5 and a strong gel with a G' of 540Pa was obtained at pH 4.6. The inclusion of 0.07%
k-carrageenan solution prevented gelation on acidification but the sample with 0.07% k-carrageenan present as microgels had similar rheolgical behaviour to the control system. This indicates that k-carrageenan microgels have the potential for use as delivery systems in yoghurt without having detrimental effects on the rheology of the yoghurt.
Contact: Dr John Mounsey;
Tel: 025 42443;
E-mail:
[email protected]
|


