Whey is a by-product of cheese and casein production and contains 15 to 20 percent of the protein in milk. The mainly globular structure of the whey proteins is sensitive to heat treatment. Upon temperature increase, these proteins unfold and immediately form large aggregates. This aggregation can, under favourable circumstances, lead to the formation of a three-
dimensional gel network. These heat-induced whey protein aggregates or gels are suitable for use as functional ingredients in the food industry, where modified texture or water-binding properties are required.
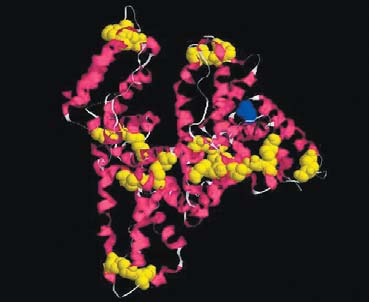 Three-dimensional structure of serum albumin showing the internal disulphide bonds (yellow) and the free sulphdryl group cys43 (blue)
It is generally believed that disulphide bonds that are formed during heat treatment function as stabilising forces in the protein gel network. In a recent study, a project was carried out in collaboration with the University of Guelph, Canada, whereby a mass spectrometry/proteomics approach was used to elucidate the molecular structure of these covalent aggregates. In the simplest case, a heat-induced dimer of the blood protein Bovine Serum Albumin (BSA) present in whey, was produced. The protein was chosen because of its abundance of internal disulphide bonds, which can act as potential precursors for inter-molecular disulphide bonds. The dimer was subjected to 'in-gel' protease digestion, which cleaves along the protein backbone leaving both inter- and intra-molecular disulphide bonds intact. The digested peptides are then analysed by Matrix Assisted Laser Desorption Ionisation – Time Of Flight – Mass Spectrometry (MALDI-TOF-MS). The experimentally determined masses were compared to peptide masses of theoretical digestions, which allowed disulphide-linked peptide dimers and trimers to be identified purely by their molecular weight. The peptide pattern was compared to that of a theoretical model of all possible disulphide bonds that could be formed between peptides. The results revealed that the most common disulphide-linked peptides involved a free sulphydryl group (here cys34), which is in agreement with the model of a sulphydryl-disulphide interchange chain reaction. It was also shown that the disulphide bonds between the proteins did not appear to be specific and a wide range of peptide combinations could be detected.
Three-dimensional structure of serum albumin showing the internal disulphide bonds (yellow) and the free sulphdryl group cys43 (blue)
It is generally believed that disulphide bonds that are formed during heat treatment function as stabilising forces in the protein gel network. In a recent study, a project was carried out in collaboration with the University of Guelph, Canada, whereby a mass spectrometry/proteomics approach was used to elucidate the molecular structure of these covalent aggregates. In the simplest case, a heat-induced dimer of the blood protein Bovine Serum Albumin (BSA) present in whey, was produced. The protein was chosen because of its abundance of internal disulphide bonds, which can act as potential precursors for inter-molecular disulphide bonds. The dimer was subjected to 'in-gel' protease digestion, which cleaves along the protein backbone leaving both inter- and intra-molecular disulphide bonds intact. The digested peptides are then analysed by Matrix Assisted Laser Desorption Ionisation – Time Of Flight – Mass Spectrometry (MALDI-TOF-MS). The experimentally determined masses were compared to peptide masses of theoretical digestions, which allowed disulphide-linked peptide dimers and trimers to be identified purely by their molecular weight. The peptide pattern was compared to that of a theoretical model of all possible disulphide bonds that could be formed between peptides. The results revealed that the most common disulphide-linked peptides involved a free sulphydryl group (here cys34), which is in agreement with the model of a sulphydryl-disulphide interchange chain reaction. It was also shown that the disulphide bonds between the proteins did not appear to be specific and a wide range of peptide combinations could be detected.
The study showed that molecular tools such as mass spectrometry combined with a proteomics style analysis can be used to reveal the underlying molecular structure of protein aggregates. This knowledge may be used to control the structure of these complex protein systems and therefore add value to food ingredients such as whey proteins.
Contact: Dr André Brodkorb;
Tel: 025 42222;
e-mail
[email protected]
|

