One of the main problems associated with current tumour
therapies is their poor discrimination between cancerous and normal cells. Thus damage to normal cells leads to numerous side effects and limits the amount of drugs that can be administered to patients. Our research at the University of Limerick is focussed on antibody-directed enzyme prodrug therapy (ADEPT), a therapeutic approach that is designed to provide site-specific delivery of cytotoxic drugs for tumour therapy.
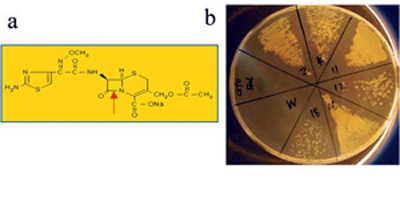 (a) structure of the cephalosporin antibiotic cefotaxime; the red arrow shows the enzyme cleavage site. (b) E. coli clones screened on cefotaxime; W is the unmutated enzyme and 2, 4, 11, 12, 16 and 18 are mutated clones with increased activity against the antibiotic.
In ADEPT, an antibody-enzyme complex that is specific for tumours is injected and allowed to localise on the tumour cell surface. This is followed by administration of a non-toxic "prodrug", which is activated at the tumour by the activity of the localised enzyme and leads to killing of the tumour cells. One of the limitations of this approach is the need for the enzyme to be both immunocompatible, ensuring it will not be rejected by the patient's immune system prior to activation of the prodrug, and to exhibit a novel enzymatic specificity � to ensure the active drug is released only at the tumour.
(a) structure of the cephalosporin antibiotic cefotaxime; the red arrow shows the enzyme cleavage site. (b) E. coli clones screened on cefotaxime; W is the unmutated enzyme and 2, 4, 11, 12, 16 and 18 are mutated clones with increased activity against the antibiotic.
In ADEPT, an antibody-enzyme complex that is specific for tumours is injected and allowed to localise on the tumour cell surface. This is followed by administration of a non-toxic "prodrug", which is activated at the tumour by the activity of the localised enzyme and leads to killing of the tumour cells. One of the limitations of this approach is the need for the enzyme to be both immunocompatible, ensuring it will not be rejected by the patient's immune system prior to activation of the prodrug, and to exhibit a novel enzymatic specificity � to ensure the active drug is released only at the tumour.
In our work, protein modelling and directed mutagenesis are utilised to generate human enzymes with activity against common penicillin and cephalosporin antibiotics, upon which many prodrugs for ADEPT are based. As beneficial mutations can be difficult to predict from protein structure, shuffling of DNA from different enzymes is also carried out in a procedure that mimics natural evolution in vitro. Mutated enzymes are produced in the bacterium Escherichia coli and clones with desired activities are identified by screening on antibiotic-containing media. The mutated proteins are purified and characterised using enzyme assays and nuclear magnetic resonance (NMR). Testing of candidate enzymes with currently available and novel prodrugs will allow identification of enzyme-prodrug combinations with the potential to provide tumour-targeted cytotoxicity in vivo.
Contact: Dr Gerard Wall,
Dept. of Chemical and Environmental Sciences and Materials and Surface Sciences Institute (MSSI),
University of Limerick;
E-mail:
[email protected]
|

