| 2005 |

|
YEAR BOOK |
University College Dublin
|
The final cut – rediscovering your inhibitions
|
HIV ('Human Immuno-deficiency Virus') is a virus that attacks the immune system of the body diminishing the immune systems ability to fight disease. At the end of 2004, it was estimated that over 39 million people worldwide had contracted the HIV/AIDS virus. Since 1981 over 20 million people have died from AIDS.
Alzheimer's disease is a progressive, irreversible brain disorder with no known cause or cure. Approximately 100,000 victims die and 360,000 new cases of Alzheimer's disease are diagnosed each year.
The connection between these two debilitating diseases lies in the large molecules that are required for the propagation of the HIV virus and the development of Alzheimer's within the body. These molecules are called proteinases and their job is to catalyse the cleavage of proteins. Proteinases are involved in many processes including digestion and blood clotting. By understanding how these molecules catalyse the breakdown or cutting up of proteins we can design other molecules that will stop or inhibit them.
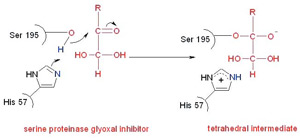
The proteinases are a large family of enzymes that include the serine, cysteine, aspartyl and metallo-proteinases. Our research group is currently looking at all four types of proteinase. My work concentrated on the serine proteinases and in particular chymotrypsin. Serine proteinases are so named because of the reactive serine residue in the catalytic site of the enzyme. This bonds directly to the substrates during catalysis. Chymotrypsin is often used as a model proteinase for developing serine proteinase inhibitors due to its availability and relative stability under experimental conditions.
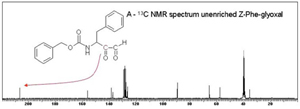
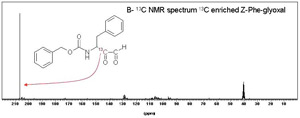
Catalysis by the serine proteinases is thought to proceed via an acyl intermediate. The build up and break down of this is via a tetrahedral intermediate. The efficiency of catalysis is thought to depend on how well the enzyme can stabilise the tetrahedral intermediate. However, there is no appreciable build up of this intermediate during catalysis and so it cannot be studied directly. Our aim was to develop inhibitors that would form stable adducts that mimic the catalytic tetrahedral intermediate. We would then use carbon-13 NMR (Nuclear Magnetic Resonance) spectroscopy to look at the enzyme inhibitor complexes. 13 C-NMR is a technique that gives a signal for every carbon atom in the molecule being examined. In small molecules containing 10-50 carbon atoms assigning individual signals is possible ( Figure 1A ) but when looking at large molecules such as enzymes with thousands of different carbon atoms, finding and studying the signals you are interested in is difficult. By enriching certain carbon atoms in our inhibitor to nearly 100% 13 C (the natural abundance of the 13 C isotope is 1%) we can increase specified signals a hundred fold and easily pick them out from the other signals.
*Edward Spink was one of the finalists in AccesScience '05; a competition for 3rd year postgraduate students held in UCD in March 2005. This is a summary of his presentation. He is a third year PhD student working in Professor Paul Malthouse's Group.
Contact: Edward Spink, Department of Biochemistry,
Centre for Synthesis and Chemical Biology, Conway Institute,
UCD, Belfield, Dublin 4;
Tel: (01) 7166838; E-mail: [email protected]