| 2005 |

|
YEAR BOOK |
University College Dublin
|
NMR based drug discovery
|
Determination of the three dimensional solution structure of polypeptides was not possible a few years back due to the lack of strong magnetic fields. Conventional analytical techniques are usually too slow and insensitive, and also require large homogeneous amounts of sample. Recent developments in the magnet technology followed by the developments in the high resolution NMR spectroscopic techniques have enabled NMR spectra to be obtained and analysed in small quantities of biologically important material. The high resolution NMR spectroscopy provides a sensitive and non-destructive analytical method for completely characterising biologically important molecules. This is also the case for the recent development of computer technology. Now we have the state-of-the-art computing facilities for interpreting solution NMR data and molecular modelling studies.
By combining these two technologies, we would be able to predict the structure related parameters of biologically important molecules. Using the NMR spectroscopy we would be able to identify all the individual amino acids and their individual atomic components of the polypeptide chain. NMR data can be used to obtain the primary and secondary structural data. These structural data along with other information can then be translated into structural parameters for molecular modelling studies. Three dimensional solution structures can be obtained after several molecular modelling steps.
Type 2 diabetes mellitus is characterised by a decreased responsiveness of peripheral tissues to insulin and a diminished and delayed pancreatic b-cell response to glucose. Novel therapeutic agents that normalise the b-cell response to glucose are therefore of considerable interest in the treatment of type 2 diabetes. One candidate agent is the gastrointestinal peptide, glucose-dependent insulinotropic polypeptide (GIP), which acts as major insulin-releasing hormone through the enteroinsular axis. To understand the structural requirements for the biological activity GIP, the solution structure was investigated by proton NMR and modelling. The structure of GIP is characterised by a well defined alpha-helical conformation between residues 6-29, and a second loose helical segment between residues 30-36 in 50% TFE/water.
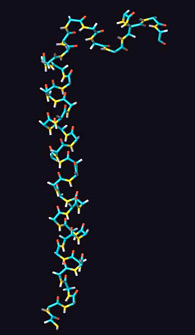
Type 2 diabetes mellitus is characterised by a decreased responsiveness of peripheral tissues to insulin and a diminished and delayed pancreatic b-cell response to glucose. Novel therapeutic agents that normalise the b-cell response to glucose are therefore of considerable interest in the treatment of type 2 diabetes. One candidate agent is the gastrointestinal peptide, glucose-dependent insulinotropic polypeptide (GIP), which acts as major insulin-releasing hormone through the enteroinsular axis. To understand the structural requirements for the biological activity GIP, the solution structure was investigated by proton NMR and modelling. The structure of GIP is characterised by a well defined alpha-helical conformation between residues 6-29, and a second loose helical segment between residues 30-36 in 50% TFE/water.
Contact: Dr. Chandralal Hewage, Department of Biochemistry,
Conway Institute of Biomolecular and Biomedical Research,
UCD, Belfield, Dublin 4;
E-mail: [email protected]; Web: http://www.ucd.ie/biochem/ch/