| 2004 |

|
YEAR BOOK |
University of Limerick
|
Life after death in the microbial world: UL develops novel Flow Cytometry methods to understand the afterlife of bacteria in foods
|
Traditionally, bacteria are deemed non-viable when cells recovered from a sample do not grow when plated onto solid agar media. However, non-viable cells impact greatly on food quality and safety either by producing flavour or generating toxins during storage.
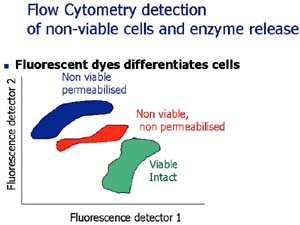
During ripening of fermented foods, Lactic acid bacteria (LAB) become non-viable. Yet their proteolytic enzymes, located inside non-viable cells, develop the characteristic flavour of foods such as Cheddar cheese. But, to be effective, these enzymes must be released into the product. Hence studying non-viable LAB will allow us to understand whether different strains remain intact, or become permeable (leaky) or autolyzed (ruptured). The balance between these different states could influence the release of enzymes, how fast a product ripens, and the type of flavour produced. In terms of food safety, pathogenic bacteria such as Bacillus cereus survive food heat treatments by forming protective spores or existing as non-viable injured cells, recovering later to grow and cause spoilage during product storage.
The problem is how to identify and count bacteria in various non-viable states. Conventional staining of bacteria with fluorescent dyes, which counts a few hundred cells using a microscope, is a haphazard way to study this complex microbial ecosystem. Flow cytometry (FCM) is an exciting technique, which may solve these limitations. FCM can analyse 10 000 stained cells in 30 seconds, grouping these cells into distinct populations based on their uptake of fluorescent dyes, which label cells based on their structure and physiology. While FCM has been developed for analysis of sea-water, soil and medical specimens, it has not yet found widespread application in food analysis.
Contact: Dr Martin Wilkinson, Department of Life Sciences, University of Limerick;
Tel: +353-61-213440; E-mail: [email protected]