| 2004 |

|
YEAR BOOK |
University of Limerick
|
The catalytic abatement of gaseous ammonia
|
Ammonia is a well-known gaseous pollutant that has several undesirable effects on the atmosphere. For example, ammonia emissions play a part in the acidification of the environment, and its precursors are responsible for the greenhouse effect and city smog. Some methods are available for controlling ammonia emissions � such as adsorption, biological purification and catalytic decomposition. Each has its disadvantages: for example, in catalytic decomposition, ammonia decomposes in the absence of oxygen into nitrogen and hydrogen using a platinum�ceramic membrane catalyst. However, temperatures of over 600 0 C are required for this process. An alternative method for ammonia destruction, which is being developed in the University of Limerick, is the catalytic oxidation of ammonia to nitrogen and water, which operates at lower temperatures (200�400 0 C).
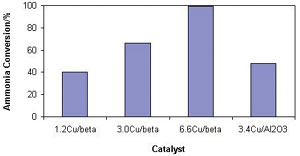
The figure presents the ammonia conversion at 300 0 C for copper exchanged beta zeolites and a copper oxide supported on alumina. Ammonia conversion increased with copper content for the copper exchanged beta catalysts. Indeed, the 6.6Cu/beta (a beta zeolite containing 6.6wt% copper) catalyst proved to be extremely active, presenting complete conversion at 300 0 C. A promising feature of all the beta catalysts was the very high selectivity to nitrogen with little or no NO and N 2 O detected. This high selectivity to nitrogen and high activity makes the copper based beta catalyst one of the most active catalysts reported to date in the literature for the oxidation of ammonia to nitrogen.
The activity of the catalysts in the presence of water has also been investigated. Water vapour does not affect the selectivity to nitrogen, although it does have an adverse effect on the overall activity. However, increasing the copper content of the beta zeolite can reduce this negative effect.
Contact: Teresa Curtin, Chemical and Environmental Sciences Department, University of Limerick;
Tel: +353-61-202981. E-mail: [email protected]