| 2004 |

|
YEAR BOOK |
University College Dublin
|
Chirality in the courtroom – the trial of an athlete
|
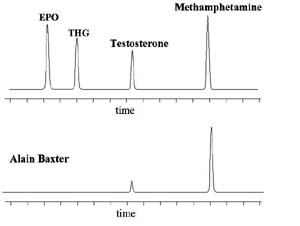
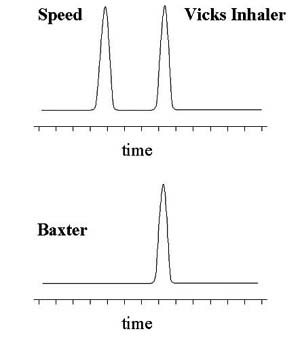
A medal winner in a recent Olympic Games event was forced to forfeit a medal when a drug test gave a positive reading for Speed. The athlete denied knowingly taking the drug, the only defence being that medication was taken to help clear a cold before competing. The rules of the International Olympics Committee, IOC, state that 'an athlete is responsible for what turns up in a drug test, regardless of how it got there'. The evidence for this case requires an understanding of the chemistry behind Speed (a stimulant drug of abuse) and the cold medication, the effect of drugs on our bodies, and the standard methods of drug testing.
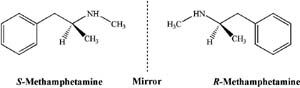
Chirality is relevant to our competitor's case as the active ingredient in the medication, S-methamphetamine, contains a chiral centre and so has a mirror image, R-methamphetamine, that is known as Speed (Figure 1). R- and S-methamphetamine have identical physical and chemical properties in the absence of an external chiral influence – e.g. they have the same melting point, chromatographic retention time, and Nuclear Magnetic Resonance spectra as each other.
However, biological systems can recognise the members of a pair of enantiomers as different substances, and the two enantiomers will therefore elicit different responses. Why do enantiomers have different biological activities? In order to exert its biological action, a chiral molecule must interact with a chiral receptor, similar to a hand fitting into a glove. But just as a right hand can only fit into a right hand glove, so a particular enantiomer can only fit into a receptor site having the complimentary shape. The other enantiomer will not fit, like a right hand in a left glove, but may fit into a receptor site elsewhere in the body and cause an unwanted effect, or may even accumulate to potentially toxic levels in the body.
Not surprisingly, the Food and Drug Administration insists that a pharmaceutical company which wishes to market a drug as a mixture of enantiomers must establish the activity of both enantiomers and also show that the unwanted enantiomer does not cause any adverse effects. The search for efficient syntheses of enantiomerically pure compounds is an active area of research in both academic and industrial laboratories. Under normal laboratory conditions, when a chiral centre is introduced into a molecule, a mixture containing equal amounts of the two enantiomers is obtained.
My area of research focuses on the use of asymmetric synthesis to selectively prepare one enantiomer. This is achieved through the introduction of a chiral ligand – a bulky molecule that influences the formation of the new chiral centre. My work focuses on the synthesis of one new class of chiral ligands, and testing their efficacy in preparing enantiopure compounds. The basic design of my ligands is very promising, as they have selectively formed 97.5% of one enantiomer and only 2.5% of the other in an important synthetic transformation.
*Theresa Ahern was one of the participants in AccesScience '04 held in UCD in May 2004. This is an edited summary of her presentation. She is a third year PhD student working with Professor Pat Guiry.
Contact: Theresa Ahern, Department of Chemistry, Centre for Synthesis and Chemical Biology,
Conway Institute, University College Dublin;
Tel: (01) 7162319; E-mail: [email protected]