| 2004 |

|
YEAR BOOK |
University College Cork, Cork University Hospital & Teagasc
|
Phage invaders – natural therapeutic agents
|

Killer phage
In the world of micro-organisms, a bacterium's deadliest enemy is a specialised virus called a phage. These tiny entities with unusually structured features have evolved into an elite and deadly attacking force. Although considerably smaller than the bacterial cells they infect, they systematically set about targeting and killing, continually increasing their number until every susceptible cell has been destroyed.
Their mode of killing is clinically efficient – initially they survey and recognise the cell to be attacked, they then conceal themselves by 'docking' on the bacterial surface, after which they inject their DNA into the unsuspecting bacteria. At this stage, the fate of the host is sealed. In a short space of time, the bacterial cell is hijacked and multiple copies of the virus are produced and assembled.
Many chronic diseases such as tuberculosis and leprosy are caused by specific types of mycobacteria. Similar mycobacterial bugs have been found in the gut of individuals suffering from Inflammatory Bowel Disease. Although their role in causing disease in humans is still unknown, their presence may prolong the condition and cause further inflammation. It is therefore wise to investigate ways of eradicating potential pathogens such as mycobacteria from the site of infection.
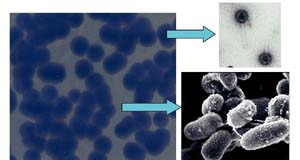
Controlling mycobacterial disease
An effective way of controlling and killing mycobacteria in the gut and elsewhere may be through the use of these bacterial viruses. At the Science Foundation Ireland funded Alimentary Pharmabiotic Centre (APC) UCC, where the characterisation and treatment of Inflammatory Bowel Disease is a major objective, initial research has demonstrated that a specific type of bacterial virus (D29) will target and kill selected strains of mycobacteria. Under optimum conditions, just 1,000 phage are needed to kill over a billion mycobacteria in under 24 hours. An advantage of this approach is the specificity of the phage – only mycobacteria are targeted; all other bacteria are excluded from the killing effects of the phage due to the specific attraction of the phage and mycobacteria.
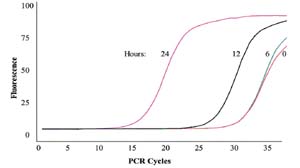
Using sophisticated real-time Polymerase Chain Reaction (PCR) technology, it is possible to monitor the increase of phage DNA as it rapidly replicates within the bacterial cells over time during infection (Figure 2). The increase in phage DNA directly reflects the corresponding increase in phage numbers and graphically shows the efficiency of these tiny infectious agents as they scavenge for unwary cells.
Genetic approach
The D29 viral genome is composed of about 49,000 base pairs. Detailed analysis has revealed a collection of genes which are known to assist the virus in exiting the bacterial cell. These genes code for a protein complex that is lethal for mycobacteria. Once synthesised during infection, their function is to punch holes in the bacterial cell wall, allowing new viruses to exit, inevitably killing the host.
Attempts are being made at the APC to transfer these genes from the virus to specialised bacteria in an effort to produce large amounts of the proteins. If successful, these proteins could be used independently of the phage as a means of controlling and eradicating mycobacterial infections.
Future prospects
Although the use of phage in treating clinical disease is an old concept dating back 100 years, its popularity almost disappeared with the discovery of antibiotics in 1928. However, in the old Soviet Union, scientists continued to study these anti-microbial agents well into the 1950s. Only recently, with the emergence of antibiotic resistant bacteria, has the potential for using phage in treating disease been re-discovered.
Although hugely effective, phage therapy has some obstacles to overcome before it can replace or complement traditional therapeutic regimes. For instance, many phage have a narrow spectrum of activity which limits their effectiveness, they may be inactivated by antibodies during treatment, and delivery of the phage to the site of infection is also an issue. However, future research may provide solutions and help to assist scientists in optimising a safe and natural resource for the treatment of clinical conditions such as inflammatory bowel disease.
|
About the APC
The Alimentary Pharmabiotic Centre (APC) is a Science Foundation Ireland Centre for Science Engineering and Technology (CSET) and is a partnership between University College Cork (UCC), Teagasc (Moorepark), and industry. The Centre's mission is to link Irish science with industry and society through excellence in research, education and outreach in gastrointestinal health. |

|

|
Contact: Dr Sally Cudmore, Manager, Alimentary Pharmabiotic Centre, Biosciences Institute, University College Cork;
E-mail: [email protected] ; Web: http://apc.ucc.ie