| 2004 |

|
YEAR BOOK |
Dublin City University & Royal College of Surgeons of Ireland
|
Thrombosis ľ a sticky business!
|
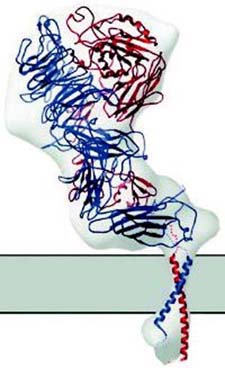
The holy grail has been to visualize single proteins within a natural or mimetic cell membrane while undergoing its normal function, e.g., undergoing a conformational change when challenged by an agonist.
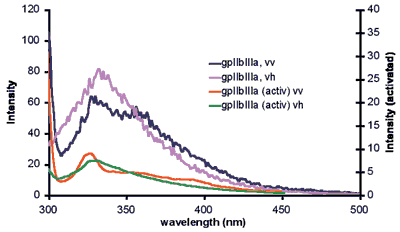
In collaboration with Dermot Kenny and Niamh Moran of the RCSI, Tia Keyes and Robert Forster of DCU are developing this 'molecular scale movie' by simultaneously observing conformational and electrical changes as agonists bind, the protein conformation changes, and the clotting cascade is initiated. To achieve this goal, we are exploring novel nanostructured cavities that uniquely enable simultaneous optical and electrochemical measurements on single proteins under well-defined agonist conditions. The team is developing techniques, such as plasmonic enhanced fluorescence and surface enhanced raman spectroscopy, that can detect conformational change. For example, fluorescence resonance energy transfer (FRET) is being exploited to visualize protein conformation change in real time. FRET involves using two dyes: a donor fluorophore transfers energy in a strongly distant and orientation-dependent fashion to an acceptor fluorophore. Hence the distance, or possibly orientation, of the two dyes, can be measured, yielding information about protein conformational changes when an agonist or therapeutic agent, e.g., aspirin, is present. Understanding the specifics of conformational change in this sticky platelet protein is critical to the development of novel therapeutic agents for the treatment of thrombosis.
Contact: Dr Tia Keyes, School of Chemical Sciences, Dublin City University, Dublin 9;
Tel. 353-1-7008185; E-mail: [email protected]