| 2004 |

|
YEAR BOOK |
Teagasc � Dairy Products Research Centre, Moorepark, Fermoy, Co. Cork
|
A membrane filtration approach to whey protein fractionation
|
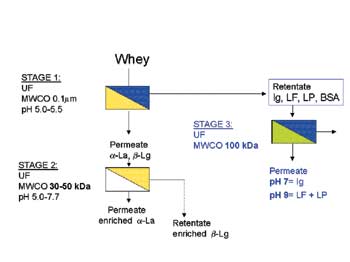
A new perspective on this emerged recently when it was shown that molecular size in addition to molecular weight may matter when it comes to determining which protein permeates a membrane of a specific molecular weight cut-off (MWCO). This is because the hydrodynamic volume of protein molecules is influenced by the surrounding charge, and has the effect of making molecules appear physically larger. Since proteins have different isoelectric points (i.e. the pH at which the surrounding charge is neutral), an opportunity is presented to select for individual fractions by differentiating on the basis of hydrodynamic volume. Solute conditions such as pH and ionic content may be adjusted in order to influence molecular charge. This concept has been demonstrated by other researchers to work satisfactorily using model solutions. However, the applicability of this concept to whey has been the subject of a study at Moorepark, where fouling phenomena add complexity to membrane separation mechanisms.
To date, a first stage membrane fractionation of the so-called antimicrobial proteins (immunoglobulins (Ig), lactoferrin (Lf)/lactoperoxidase) has been successfully accomplished. The resulting permeate containing remaining fractions has been the focus of a second stage membrane filtration for the separation of a-lactalbumin from �-lactoglobulin. A range of membranes of different MWCO and varying processing conditions have been under evaluation. Results to date suggest that it is possible to achieve a-lactalbumin/�-lactoglobulin enrichment levels of greater than two.
Contact: Dr Raj Mehra, Dr Phil M. Kelly; Tel: 025 42433;
E-mail: [email protected]