The kidneys perform the vital function in the body of constantly working to remove waste products from the blood. These include both foreign substances such as drugs and toxins as well as physiologically occurring wastes created by the body performing its various day to day functions. This constant filtering carried out by the kidneys is so critical to maintaining homeostasis or balance in the body that the kidneys receive 25% of the blood from every heart beat, managing to filter 190L of blood every 24 hours. Without the purifying actions of the kidneys these harmful products in the blood would quickly build up to a lethal level.
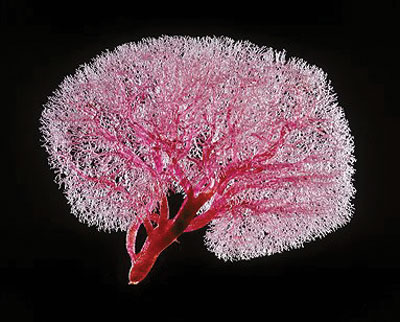 Resin cast showing the extensive network of nephrons.
The functional units of the kidney are the nephrons and, each kidney contains one million nephrons. It is into these tiny convoluted tubules, which run throughout the kidney that the blood is filtered. The cells, which make up the wall of the tubule, function to reabsorb any reusable molecules from the filtered blood while allowing any unneeded waste products to pass into the urine and be eliminated from the body. In kidney disease, or kidney fibrosis, the kidney becomes scarred and begins to malfunction. Initiating causes for kidney disease are varied but include diabetes, hypertension and drug toxicity among others. It has become apparent that, regardless of the initiating factor of the disease, the kidney seems to go through the same pattern of deterioration. Excess amounts of protein begin to accumulate in the spaces between the tubules. This swelling causes the tubules to be "squashed", which results in the tubules becoming blocked and unable to carry out their normal function of filtering and cleaning the blood; much like a sieve when it becomes clogged. This process continues until the kidney can no longer sustain life. At that point, the only options for the patient are either dialysis or transplantation, both of which are invasive and costly and neither address the cause of the disease itself. In the picture above, we can see a resin cast of the urinary tubules in the kidney showing the extensive network of nephrons and larger collecting ducts that permeates the kidney. It has been observed that damage to the kidney and in particular to these tubules, can cause it to be invaded by the body's own defensive white blood cells as they attempt to address the progressing disease. However, sometimes these white blood cells cause more harm than good.
Resin cast showing the extensive network of nephrons.
The functional units of the kidney are the nephrons and, each kidney contains one million nephrons. It is into these tiny convoluted tubules, which run throughout the kidney that the blood is filtered. The cells, which make up the wall of the tubule, function to reabsorb any reusable molecules from the filtered blood while allowing any unneeded waste products to pass into the urine and be eliminated from the body. In kidney disease, or kidney fibrosis, the kidney becomes scarred and begins to malfunction. Initiating causes for kidney disease are varied but include diabetes, hypertension and drug toxicity among others. It has become apparent that, regardless of the initiating factor of the disease, the kidney seems to go through the same pattern of deterioration. Excess amounts of protein begin to accumulate in the spaces between the tubules. This swelling causes the tubules to be "squashed", which results in the tubules becoming blocked and unable to carry out their normal function of filtering and cleaning the blood; much like a sieve when it becomes clogged. This process continues until the kidney can no longer sustain life. At that point, the only options for the patient are either dialysis or transplantation, both of which are invasive and costly and neither address the cause of the disease itself. In the picture above, we can see a resin cast of the urinary tubules in the kidney showing the extensive network of nephrons and larger collecting ducts that permeates the kidney. It has been observed that damage to the kidney and in particular to these tubules, can cause it to be invaded by the body's own defensive white blood cells as they attempt to address the progressing disease. However, sometimes these white blood cells cause more harm than good.
My work involves the isolation of white blood cells from fresh blood samples. These isolated cells are then cultured in the laboratory and activated. Products released by these activated white blood cells are then collected and introduced to normal kidney tubular cells. By addition of these products to our normal kidney cells, we hope to mimic the molecular "talk" between these two cells during kidney disease and determine the resultant effects.
Through our studies, we have determined that the interaction between the white blood cells and the kidney's own tubular cells causes a change in cell type within the kidney. Cells, which normally form a functioning part of the tubules within the kidney, undergo a change into cells that produce more proteins; thus adding to the progression of the disease. At the most basic level, the cells change shape from cuboidal compact kidney cells to more elongated cell types called fibroblasts. These fibroblasts produce more of a protein called fibronectin, which is one of the main proteins involved in renal disease.
Fibronectin occurs normally in the kidney and acts like a scaffold onto which the tubules are built. In the picture below, we can see fibronectin (stained in green) arranged in rings around the kidney cells (stained in red). However, an excess of this protein can crowd and squash the tubules causing interference with their normal activities, as happens in kidney disease.
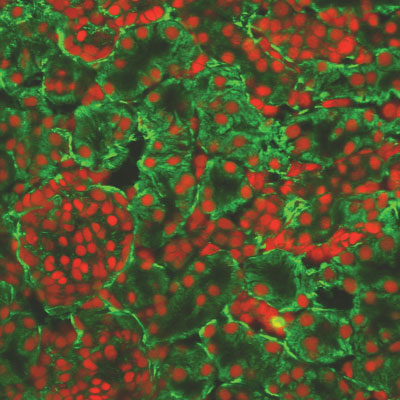 Fibronectin, stained green, supporting the kidney cells, stained red.
Like many disorders that occur in the body, kidney disease is marked by an imbalance. Through our research, we have isolated one of the proteins whose regulation is affected by the invasion of the white blood cells. We also noticed a stark change in cell shape and an increased ability of the cells to move. We think that all these factors combined strongly contribute to the deterioration of the kidney's tubules observed during kidney disease. There is a great need for further understanding of kidney disease. At the moment, there is no adequate therapy to address the progression of the disease and we hope that our research will contribute to the larger body of work ongoing with the aim of finding new treatments for the disease.
Fibronectin, stained green, supporting the kidney cells, stained red.
Like many disorders that occur in the body, kidney disease is marked by an imbalance. Through our research, we have isolated one of the proteins whose regulation is affected by the invasion of the white blood cells. We also noticed a stark change in cell shape and an increased ability of the cells to move. We think that all these factors combined strongly contribute to the deterioration of the kidney's tubules observed during kidney disease. There is a great need for further understanding of kidney disease. At the moment, there is no adequate therapy to address the progression of the disease and we hope that our research will contribute to the larger body of work ongoing with the aim of finding new treatments for the disease.
*Stephen Nolan, Conway Integrative Biology, won first place in AcessScience 'O6, a competition for 3rd year post-graduates held in UCD in March 'O6. This is a summary of his presentation.
Contact: Stephen Nolan,
UCD School of Biomolecular & Biomedical Research, and UCD Conway Institute,
University College Dublin, Belfield, Dublin 4
E-mail:
[email protected]
Web:
www.ucd.ie/conway
|


