| 2006 |

|
YEAR BOOK |
Queen's University Belfast
|
Centre for Cancer Research & Cell Biology
|
The primary objective of the CCRCB, under the leadership of Professor Patrick Johnston, has been "to create a world-class Centre for Cancer Research and Cell Biology at QUB that will be recognised for its excellence by the international scientific community". CCRCB will ensure that Northern Ireland contributes in a strategically important way to future UK and international research initiatives in Cancer as well as Infection & Immunity and Medicinal Chemistry. A key element of this project has been the provision of a new building with state of the art laboratories and enhanced key enabling technologies. The 4,500m 2 building, which is due for completion in April 2007, has been designed primarily as a generic research facility, and will bring key research groups into a single location. With the new CCRCB we have created the first comprehensive cancer centre in Ireland � promoting international, high quality research programmes linking the University, the Health Service and other funders of cancer research.

The Centre has also recently been selected as one of the Experimental Cancer Medicine Centres for the UK and through that mechanism there are a number of ongoing projects in collaboration with colleagues in Cambridge, UCL, University of Newcastle and the Institute for Cancer Research. The Centre's aims in experimental medicine are to drive the development of phase I and II trials, to facilitate the translation of pre-clinical ideas into the clinical arena and back, and to co-ordinate the collection of clinical samples. Two Cancer Research UK first-in-human (FIH) phase I trials, the first such studies in cancer on the island of Ireland, have recently been completed in Belfast and have led to the development of phase II/III programmes. Another exciting FIH phase I study exploring nano-technology drug delivery has just opened and will add to our ever increasing activity in early clinical trials.
Emanating from the Support Programme for University Research (SPUR) initiative and the University's strategic plans for the medical Faculty, the CCRCB has gone through a period of significant expansion with recruitment of a number of senior academics, from the United States, Canada and Europe. A key appointment in July 2006 to the Chair in Cancer Research & Cell Biology has been Professor Dennis McCance (Figure 2), from the University of Rochester Medical Center.

A brief outline of Professor McCance's research is given below:
Role of the retinoblastoma protein (pRb105) and AKT in epithelial cell cycle control and differentiation
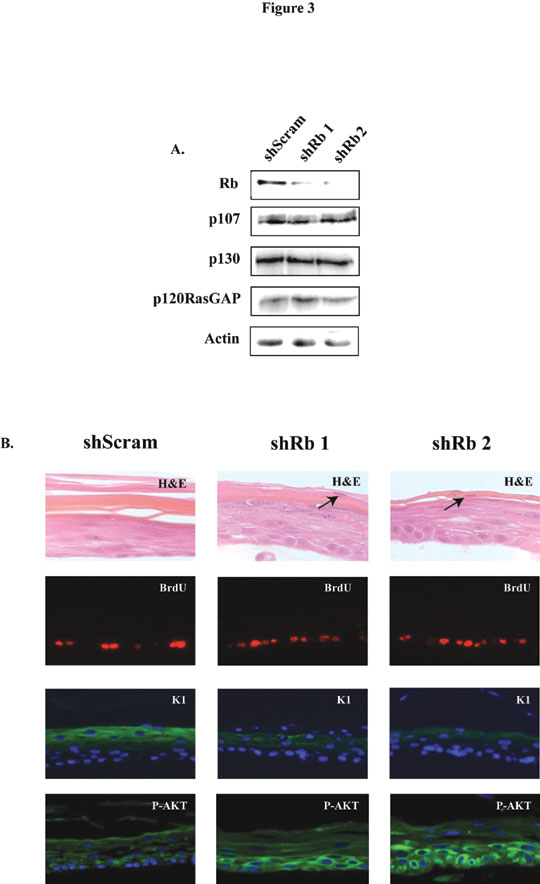
The research interests of the laboratory centre on the role of pRb105 in keratinocyte differentiation and the pathways controlled by this protein. The pRb105 pathway is disrupted in a number of cancers and the role during the cell cycle has been extensively studied. However, its role in normal cell differentiation is unclear. Human papillomaviruses (HPV) are small DNA tumor viruses causally associated with cervical cancer. The early gene product E7 from high-risk HPV is considered the major transforming protein expressed by the virus. Although many functions have been described for E7 in disrupting normal cellular processes, we have recently determined a new cellular target in primary human keratinocytes (HFK), namely, the serine/threonine kinase AKT. Expression of HPV type-16 E7 in HFK caused inhibition of differentiation, hyper-proliferation, and upregulation of AKT activity in organotypic raft cultures. The ability of E7 to up-regulate AKT activity is dependent on it binding to and inactivating the pRb105 family of proteins. Furthermore, we show that knocking down pRb105 alone, with short-hairpin RNAs (shRNAs), is sufficient to up-regulate AKT activity in differentiated keratinocytes (Figure 3). Upregulation of AKT activity and loss of pRb105 was also observed in HPV-positive cervical high grade squamous intraepithelial lesions (HSIL), when compared to normal cervical tissue. Together, these data provide evidence linking inactivation of pRb105 by E7 in the upregulation of AKT activity during cervical cancer progression. Development of AKT inhibitors may therefore help in the treatment of cancers associated with HPV infection.
Role of p63 isoforms in keratinocyte differentiation
There are 6 isoforms of the p63 family and they have high sequence homology to both the p53- and p73- family of proteins. Some of the p63 isoforms are up-regulated in epithelial cancers and this inappropriate expression is thought to play a role in malignant conversion. Using mice null for the p63 family, two groups have shown that p63 is required for skin development, although the role of each family member is unclear. Using RNAi technology we are investigating the role of each family member in keratinocyte differentiation.
Genomic instability in keratinocytes caused by HPV-16 E6 and E7
The oncoproteins, E6 and E7 from HPV-16, cause polyploidy in primary human keratinocytes within 48 hours of expression. The polyploidy cells remain stable for many passages but eventually aneuploidy cells appear. We are investigating the cellular alterations which lead to polyploidy and aneuploidy by a variety of methods, including live imaging of cells, in collaboration with Dr Conly Rieder, Wadsworth Center, Albany, NY.
For further information, please refer to the CCRCB web site: www.qub.ac.uk/ccrcb or contact:
Professor Patrick Johnston,
Scientific Director, Centre for Cancer Research & Cell Biology,
Queen's University Belfast, University Floor, Belfast City Hospital, Belfast BT9 7AB
Tel: 028 9026 3911.