| 2003 |

|
YEAR BOOK |
National University of Ireland, Galway
|
Sulfamate esters - a renaissance in interest
|

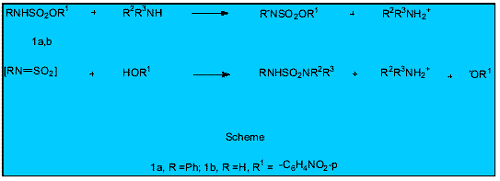
Surprisingly the mode of action of these compounds is not well understood other than to say that they clearly inhibit enzymatic action. There has been speculation about the way in which this occurs: a fuller understanding will help the design of new and better inhibitors.
In the Chemistry Department at NUI, Galway, the mechanism(s) involved has been under study for some years using esters 1a and 1b as models for the active substrates, and aminolysis as a model for a sulfamoylation reaction (see Scheme ). Based on the use of a variety of physical organic techniques, the weight of evidence supports an elimination (E-type) mechanism such as the one shown in the Scheme where clearly -OR 1 and [RN=SO 2 ] species are being eliminated in the reaction pathway. The amines do the aminolysis function by removing the NH proton of 1a/1b and they are said to act as base catalysts for the reaction. An alternative mechanism involving (nucleophilic) attack by these amines at the sulfur atom of 1a/1b (known as an S N 2(S) mechanism) appears to be excluded on the basis of mechanistic studies.
The next step in Galway is to apply these physical organic methods to probe the mechanisms of reaction of these and other esters which show high activity. It is hoped that these studies will lead to more effective treatments for breast cancer and ocular diseases.
Contact: Prof William J. Spillane; E-mail: [email protected]