| 2003 |

|
YEAR BOOK |
Teagasc - Dairy Products Research Centre, Moorepark, Fermoy, Co. Cork
|
The use of Transglutaminase in food products
|
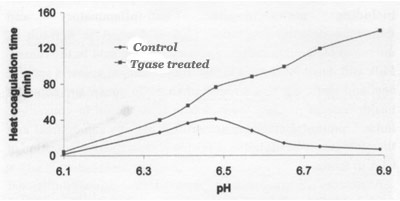
Studies carried out at Moorepark suggested that pre-incubation of different food systems with Tgase had significant effects on the subsequent behaviour of the product. Preliminary investigations of Tgase effects on skim milk suggested that intra-micellar cross-linking was not a major occurrence, no change in casein micelle size distribution parameters being observed by Dynamic Light Scattering.
Incubation of skim milk with Tgase after pre-heating at 120�C x 2 minutes and subsequent evaporation and drying resulted in a marked increase in the heat stability of the milk protein, especially at more alkaline pH (> 6.7), when the powder was reconstituted to 20% solids. This was interpreted as an inter-micellar protein crosslinking resulting in an inhibition of casein dissociation at high temperatures (120�C).
The strength of acid gels produced from casein dispersions was dramatically increased when Tgase was present during the slow acidification step. It was concluded that changes in the casein micellar structure during acidification facilitated the cross-linking function of Tgase. When ice-cream formulations were pre-incubated with Tgase, prior to pasteurisation of the mix and subsequent freezing, the melting profile of the frozen ice-cream was variably affected depending on the time of incubation and the enzyme concentration. It was concluded that controlled partial cross-linking of casein aggregates was necessary to enhance this particular attribute in ice-cream.
The water-binding capacity of sodium caseinate, a commodity milk protein ingredient could be increased 5-10 times by judicial treatment of the skim milk prior to processing. This involved close control on the partial cross-linking process. While this work is still ongoing, it was evident that strict control of the degree and type of Tgase-induced protein cross-linking was required to maximise any beneficial effects in food.
Contact: Dr Brendan O'Kennedy; Tel: 025 42446;
E-mail: [email protected]